Structural Polymorphism and Functional Differentiation of Actin Filaments
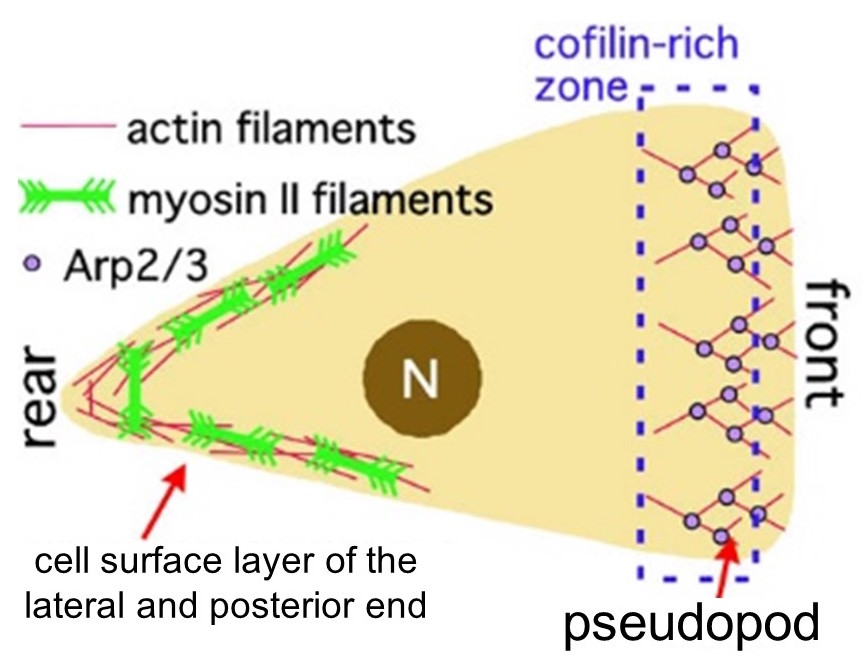
Actin filament plays a very important role in various biological processes, such as eukaryotic cell motility and intracellular transport. For instance, during amoeboid movement, the elongation of actin filament at the anterior end of the amoeba propels the pseudopod forward and actin filament interacts with myosin II at the posterior end to contract the trailing edge (Fig. 1). In such ways different structures in the cell coexist with actin filament to carry out important functions. Functional differences of actin filaments is commonly thought to be based on the differences in interacting proteins. For example, actin filaments in the anterior end of the cell polymerize in an Arp 2/3-dependent manner and depolymerization is catalyzed by cofilin. At the posterior end, myosin II filaments and filamins are bound to the actin meshwork, whose interactions are responsible for contraction.
So how do individual actin filaments bind to the appropriate actin binding protein (ABP)? As for this, there is a possibility that it is influenced by the differences in higher order structures of actin filaments (e.g. dendritic structure and mesh structure), and there are also situations where it is assumed that the binding to ABPs are controlled by a localized biochemical cell-signaling system (such as Rho activation or Ca ion increase). However, there are many cases where the above does not apply to certain ABPs, thus the entire picture of the differentiation mechanism of actin filaments remain unclear.
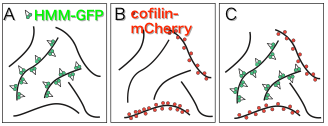
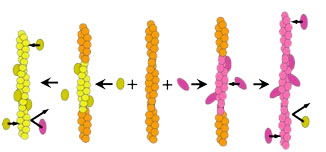
On the other hand, structural analysis of actin filaments has greatly advanced in recent years, but actin filaments have polymorphisms and it has been proposed not to assume a single structure (Galkin et al., 2010). In regards to this, we found that when actin filaments and a trace amount of HMM (a soluble fragment of myosin II) are mixed in vitro in the presence of ATP, it yielded both, sparsely HMM bound filaments and filaments with hardly bound HMM(Fig. 2A:Tokuraku et al., 2009I). There are two possible explanations for this phenomenon: 1) Actin filaments have cooperative structural polymorphism (eg., Egelman and Orlova, 1995), some of the multiple equilibrium structural states had higher affinity for HMM; 2) When HMM binds to actin filaments, it causes a cooperative structural change in actin filaments (eg., Oosawa et al., 1972), so when a molecule of HMM binds to an actin filament, structural change enhancing the affinity to HMM is propagated to the surrounding actin subunits and promotes the binding of another HMM molecule, resulting in an entire filament shifting to a high affinity state for HMM in a domino-effect manner (Fig. 3). In either case, it is suggested that actin filaments have (at least) two different structures with different affinities for HMM.
Since the binding of cofilin and actin filament is also cooperative(Fig. 2B: Galkin et al., 2001), it can also be considered that there are actin filament structures that have higher affinity for cofilin and those that have lower affinity for cofilin. In fact, we recently found that when HMM and cofilin are in the presence of excess actin filaments, HMM and cofilin each cooperatively bind to actin filaments in a mutually exclusive manner(Kijima et al., 2011 Biophysical Society; Fig. 2C). This result suggests that filaments with high affinity for HMM have low affinity for cofilin and conversely, filaments with low affinity for HMM have high affinity for cofilin. The results above have led to the consideration that the structure of actin filaments differs depending on the location in the cell, and the various actin-binding proteins such as cofilin, myosin II, and the like, may be attributed to the different intracellular localization of the filaments (Hypothesis 1).
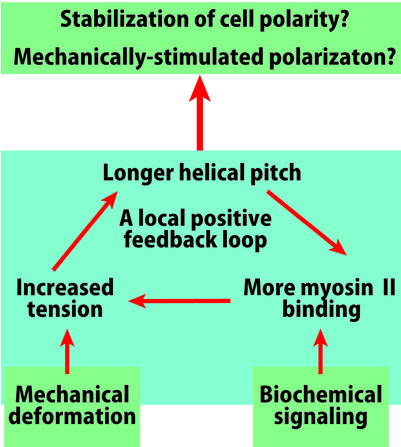
The interaction between actin filaments and myosin II at the rear of the cell produces tension, and it is also known that pulling the actin filament causes the helical pitch to elongate (Wakabayashi et al., 1994; Matsushita et al., 2011). Hence, it is expected that upon tension generation, pitch elongation and increase in the amount of myosin II binding will form a local positive feedback loop, stabilizing the contractile state at the rear end of the cell(Fig. 4). In contrast, at the anterior part of the cell, polymerization of actin filaments pushes the temporal foot forward, applying compressive force to the filament. In addition, cofilin localized in the anterior part of the cell tends to bind to non-tensioned filaments (Hayakawa et al., 2011), and cofilin binding shortens the helical pitch (McGough et al., 1999). A local positive feedback loop, which includes shortening of helical pitch, could establish at the anterior of the cell. Hence, there is a possibility that cell polarity is stabilized via structural polymorphism of actin filament (Hypothesis 2). The activation for the different positive feedback loops between the anterior and posterior end of the cell can be assumed to be controlled by biochemical signals and stochastic fluctuation, but cell transfiguration caused by mechanical stimulus can also be a trigger (Fig. 4). It is indeed known that when a localized area of a cell is transfigured by an external stimulus, myosin II accumulates to the stimulated area and cell polarity is formed at the rear side (e.g., Verkhovsky et al., 1999).
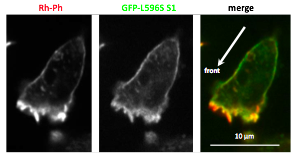
In order to verify these hypotheses, we observed the binding of actin filaments to the myosin II motor region in the cells. As expected, we found that actin filaments with applied tension had a strong affinity for myosin II motor region (Fig. 5: Uyeda et al., 2011). This observation supports hypotheses 1 and 2 above. However, the possibility that this phenomenon is a result of changes in the orientation of actin filaments due to tension is not being excluded. Thus, we are preparing to perform an in vitro verification to prove that the changes in the atomic structure of actin filament such as elongation of helical pitch increases binding of myosin II motor region.
In the explanation above, we have given examples of cooperative structural changes of actin filaments by myosin II and cofilin. Likewise, it is becoming apparent that other actin-binding proteins also cause characteristic structural changes in actin filaments. These actin-binding proteins each induce characteristic cooperative structural changes of actin filaments, which could possibly be related to functional differentiation of actin filaments. Accordingly, we are going to verify the extent to which the association between the cooperative structural change of filaments and functional differentiation of such filaments is a general phenomenon, using various actin-binding proteins.